Atoms and Molecules

Imagine you have a huge boulder in front of you, and you keep breaking it into smaller and smaller pieces. At some point, you’ll reach a stage where the particle becomes so small that it can no longer be divided further without losing its properties. This smallest indivisible particle is what we call an atom. Today, we’ll discuss the concept of atoms, molecules, laws governing their combinations, and how these tiny particles form the basis of all matter around us.
Laws of Chemical Combination
Before we dive into the concepts of atoms and molecules, let’s first understand the rules that govern how substances combine chemically. These rules are called the Laws of Chemical Combination.
1. Law of Conservation of Mass
This law states that mass can neither be created nor destroyed during a chemical reaction.
Explanation:
For example, if hydrogen reacts with oxygen to form water, the total mass of hydrogen and oxygen before the reaction will be equal to the mass of water after the reaction.
Mathematically:
Mass of reactants = Mass of Products
Law of Definite Proportions
This law states that, ” a chemical compound always contains the same elements in the same proportion by mass, irrespective of the source or method of preparation.”
Example:
Water (H₂O) will always contain 2 parts hydrogen and 16 parts oxygen by mass, no matter where you get the water from.
What Are Atoms?

Now, let’s dive deeper into atoms. Atoms are the basic building blocks of matter. Everything around us, from the air we breathe to the food we eat, is made up of atoms. But remember, atoms are indivisible in chemical processes—meaning you can’t break them down further by chemical means.
Key Features of Atoms:
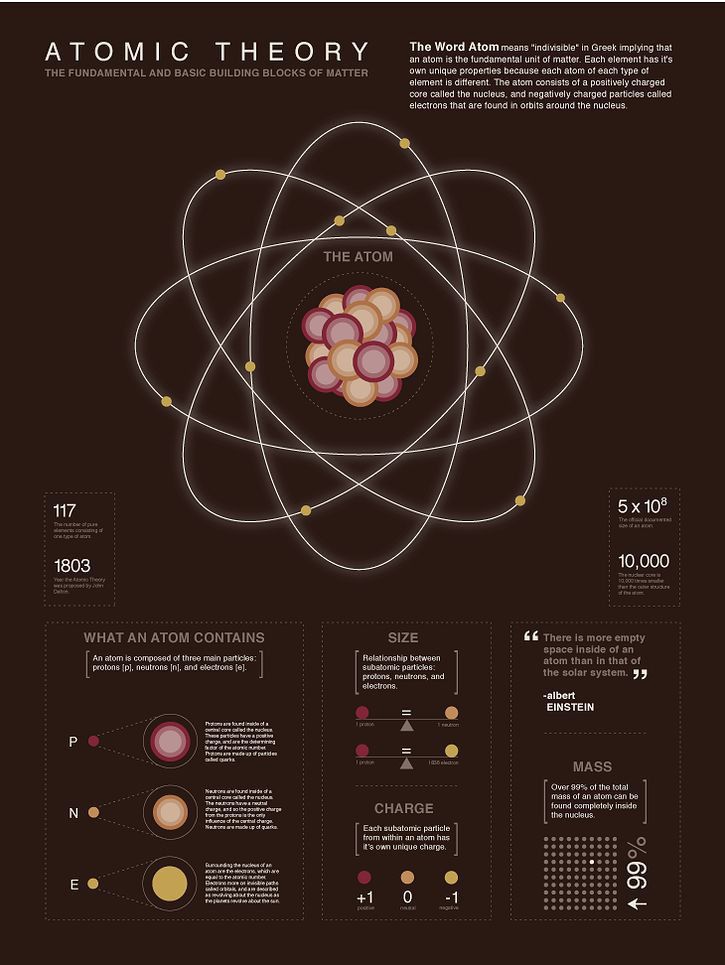
The atom is the fundamental unit of matter, retaining the characteristics of an element. Below we will know about its key features:
1. Smallest Unit of Matter:
An atom represents the tiniest division of matter that still maintains the properties of a specific element.
2. Subatomic Particles:
Protons: Positively charged particles found in the nucleus.
Neutrons: Neutral particles also located in the nucleus.
Electrons: Negatively charged particles that orbit the nucleus in specific energy levels.
3. Nucleus:
The dense center of an atom containing protons and neutrons.
It accounts for most of the atom’s mass.
4. Electron Arrangement:
Electrons are arranged in defined shells or energy levels around the nucleus, labeled as K, L, M, etc.
5. Neutrality:
In a neutral atom, the number of protons equals the number of electrons, resulting in no overall charge.
6. Atomic Number (Z):
This is the total number of protons in an atom’s nucleus, uniquely identifying each element.
7. Mass Number (A):
The mass number is the sum of protons and neutrons, representing the atom’s approximate mass.
8. Isotopes:
Atoms of the same element with identical atomic numbers but varying mass numbers due to differences in the number of neutrons.
9. Chemical Behavior:
The chemical properties of an atom are influenced by the electrons in its outermost shell, also known as valence electrons.
10. Quantum Mechanics:
The behavior and position of electrons follow quantum principles, described as regions of probability called orbitals.
11. Conservation in Reactions:
During chemical reactions, atoms are neither created nor destroyed but are simply rearranged to form new substances.
We can summarise these above like as follows below :-
1. Atoms are extremely small and cannot be seen with the naked eye.
2. They combine to form molecules or compounds.
3. Each element has its unique type of atom.
Symbols of Atoms
Each atom is represented by a specific symbol. For example:
Hydrogen: H
Oxygen: O
Sodium: Na
Chlorine: Cl
Atoms & their Symbols
Here is a list of elements with their atomic number and symbols from 1 to 118:
1. H – Hydrogen
2. He – Helium
3. Li – Lithium
4. Be – Beryllium
5. B – Boron
6. C – Carbon
7. N – Nitrogen
8. O – Oxygen
9. F – Fluorine
10. Ne – Neon
11. Na – Sodium
12. Mg – Magnesium
13. Al – Aluminium
14. Si – Silicon
15. P – Phosphorus
16. S – Sulfur
17. Cl – Chlorine
18. Ar – Argon
19. K – Potassium
20. Ca – Calcium
21. Sc – Scandium
22. Ti – Titanium
23. V – Vanadium
24. Cr – Chromium
25. Mn – Manganese
26. Fe – Iron
27. Co – Cobalt
28. Ni – Nickel
29. Cu – Copper
30. Zn – Zinc
31. Ga – Gallium
32. Ge – Germanium
33. As – Arsenic
34. Se – Selenium
35. Br – Bromine
36. Kr – Krypton
37. Rb – Rubidium
38. Sr – Strontium
39. Y – Yttrium
40. Zr – Zirconium
41. Nb – Niobium
42. Mo – Molybdenum
43. Tc – Technetium
44. Ru – Ruthenium
45. Rh – Rhodium
46. Pd – Palladium
47. Ag – Silver
48. Cd – Cadmium
49. In – Indium
50. Sn – Tin
51. Sb – Antimony
52. I – Iodine
53. Xe – Xenon
54. Cs – Cesium
55. Ba – Barium
56. La – Lanthanum
57. Ce – Cerium
58. Pr – Praseodymium
59. Nd – Neodymium
60. Pm – Promethium
61. Sm – Samarium
62. Eu – Europium
63. Gd – Gadolinium
64. Tb – Terbium
65. Dy – Dysprosium
66. Ho – Holmium
67. Er – Erbium
68. Tm – Thulium
69. Yb – Ytterbium
70. Lu – Lutetium
71. Hf – Hafnium
72. Ta – Tantalum
73. W – Tungsten
74. Re – Rhenium
75. Os – Osmium
76. Ir – Iridium
77. Pt – Platinum
78. Au – Gold
79. Hg – Mercury
80. Tl – Thallium
81. Pb – Lead
82. Bi – Bismuth
83. Po – Polonium
84. At – Astatine
85. Rn – Radon
86. Fr – Francium
87. Ra – Radium
88. Ac – Actinium
89. Th – Thorium
90. Pa – Protactinium
91. U – Uranium
92. Np – Neptunium
93. Pu – Plutonium
94. Am – Americium
95. Cm – Curium
96. Bk – Berkelium
97. Cf – Californium
98. Es – Einsteinium
99. Fm – Fermium
100. Md – Mendelevium
101. No – Nobelium
102. Lr – Lawrencium
103. Rf – Rutherfordium
104. Db – Dubnium
105. Sg – Seaborgium
106. Bh – Bohrium
107. Hs – Hassium
108. Mt – Meitnerium
109. Ds – Darmstadtium
110. Rg – Roentgenium
111. Cn – Copernicium
112. Nh – Nihonium
113. Fl – Flerovium
114. Mc – Moscovium
115. Lv – Livermorium
116. Ts – Tennessine
117. Og – Oganesson
118. Uuo – Ununoctium (now known as Oganesson, Og)
These elements make up the current periodic table from Hydrogen (1) to Oganesson (118).
The symbols are derived from either the English names or the Latin names of the elements (e.g., Sodium is , from its Latin name Natrium).
Atomic Mass
Let’s discuss atomic mass, which is one of the fundamental properties of an atom. It tells us how heavy an atom is compared to the mass of a hydrogen atom.
Atomic mass is expressed in atomic mass units (amu).
1 amu is defined as {1}/{12}th of the mass of a carbon-12 atom.
For example:
Atomic mass of oxygen = 16 amu
Atomic mass of hydrogen = 1 amu
What Are Molecules?

When two or more than two similar type of atoms combine together so it forms a molecule
Atoms rarely exist independently. Instead, they combine to form molecules, which are groups of two or more atoms chemically bonded together. Molecules can be of two types:
1. Molecules of Elements:
Molecules consisting of the same type of atoms.
Examples:
O₂ (oxygen gas)
H2 (hydrogen gas)
2. Molecules of Compounds:
Molecules consisting of different types of atoms.
Examples:
H₂O (water)
CO₂ (carbon dioxide)
Molecular structure
Molecular Structure refers to the arrangement of atoms within a molecule, including the number of atoms, the types of bonds between them (single, double, or triple bonds), and the 3D shape of the molecule. It is determined by the molecule’s chemical formula and bonding properties, which influence its physical and chemical behavior.
Examples of Molecular Structures

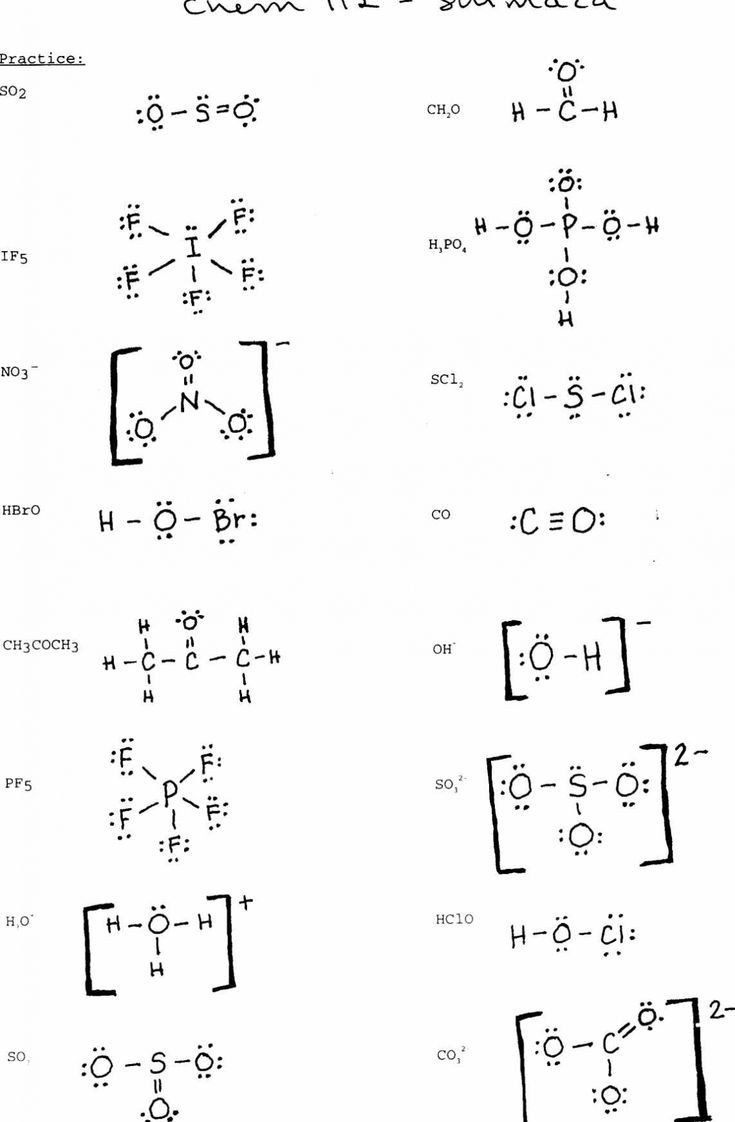
1. Water (H₂O)
Structure: Bent shape due to the two lone pairs on oxygen.
Bond Angle: ~104.5°.
Explanation: Oxygen forms covalent bonds with two hydrogen atoms, and the lone pairs create a dipole moment.
2. Carbon Dioxide (CO₂)
Structure: Linear shape.
Bond Angle: 180°.
Explanation: Carbon is bonded to two oxygen atoms via double bonds, arranged symmetrically.
3. Methane (CH₄)
Structure: Tetrahedral shape.
Bond Angle: 109.5°.
Explanation: Carbon forms single covalent bonds with four hydrogen atoms, creating a symmetric 3D structure.
4. Ammonia (NH₃)
Structure: Trigonal pyramidal shape.
Bond Angle: ~107°.
Explanation: Nitrogen forms three covalent bonds with hydrogen atoms and has one lone pair.
5. Ethanol (C₂H₅OH)
Structure: Contains a hydroxyl group (-OH) and an ethyl group (-C₂H₅).
Explanation: Hydrogen bonding from the hydroxyl group impacts its properties.
6. Benzene (C₆H₆)
Structure: Planar, cyclic structure with alternating double bonds (resonance structure).
Bond Angle: 120° for all carbon atoms.
Explanation: Aromatic compound with delocalized π-electrons.
Importance of Molecular Structure
Chemical Reactivity: Determines how molecules interact in chemical reactions.
Physical Properties: Affects boiling/melting points, solubility, etc.
Biological Function: Influences how molecules behave in biological systems, e.g., enzyme-substrate interactions.
Chemical Formulae
To represent molecules, we use chemical formulae. A chemical formula is a symbolic representation of a molecule, showing the types and numbers of atoms involved.
Rules for Writing a Chemical Formula:
1. The valency of each element is considered. Valency is the combining capacity of an element.
2. The symbols of elements are arranged in a specific sequence (usually metals before non-metals).
For example:
Water: H₂O
Ammonia:NH3
Mole Concept
One of the most powerful tools in chemistry is the mole concept. It helps us count atoms and molecules, which are incredibly small in size.
What is a Mole?
A mole is a quantity that contains particles (atoms, molecules, or ions). This number is called Avogadro’s number.
Molar Mass:
The mass of 1 mole of a substance is its molar mass.
For example:
Molar mass of oxygen (O₂) = 32 g/mol
Molar mass of water (H₂O) = 18 g/mol
What is Empirical Formula
The empirical formula of a compound shows the simplest ratio of atoms of each element in that compound. It does not give the exact number of atoms but tells us the basic proportion of elements.
For example:
If a compound has 2 hydrogen atoms for every 1 oxygen atom, its empirical formula is H₂O.
Key Points:
- Simplest Ratio: It only tells the simplest whole number ratio of elements, not the actual number of atoms.
- Different from Molecular Formula: The molecular formula shows the actual number of atoms, while the empirical formula simplifies it.
Examples:
- Glucose
Molecular formula: C₆H₁₂O₆
Empirical formula: CH₂O (because 6:12:6 simplifies to 1:2:1).
- Hydrogen Peroxide
Molecular formula: H₂O₂
Empirical formula: HO (because 2:2 simplifies to 1:1).
- Ethene (C₂H₄)
Molecular formula: C₂H₄
Empirical formula: CH₂ (because 2:4 simplifies to 1:2).
- Water
Molecular formula: H₂O
Empirical formula: H₂O (already in simplest ratio).
How to Find the Empirical Formula:
- Find the ratio of elements in the compound.
- Simplify the ratio to the smallest whole numbers.
Example:
If a compound contains 4 grams of hydrogen and 32 grams of oxygen:
Find the ratio using their atomic masses:
Hydrogen (H) = 1 g/mol, Oxygen (O) = 16 g/mol.
Moles of H = 4/1 = 4, Moles of O = 32/16 = 2.
Ratio of H:O = 4:2 = 2:1.
Empirical formula = H₂O.
Here are some practice problems related to empirical formula, along with solutions to help students understand:
Problem 1:
A compound contains 40% carbon, 6.7% hydrogen, and 53.3% oxygen by mass. Find its empirical formula.
(Atomic masses: C = 12, H = 1, O = 16)
Solution:
- Convert percentages to moles:
Moles of C = 40/12 = 3.33
Moles of H = 6.7/1 = 6.7
Moles of O = 53.3/16 = 3.33
- Divide by the smallest value (3.33):
C: 3.33/3.33 = 1
H: 6.7/3.33 = 2
O: 3.33/3.33 = 1
- Empirical formula: CH₂O
Problem 2:
A compound is found to contain 70% iron (Fe) and 30% oxygen (O) by mass. Find its empirical formula.
(Atomic masses: Fe = 56, O = 16)
Solution:
- Convert percentages to moles:
Moles of Fe = 70/56 = 1.25
Moles of O = 30/16 = 1.875
- Divide by the smallest value (1.25):
Fe: 1.25/1.25 = 1
O: 1.875/1.25 = 1.5
- Multiply to get whole numbers:
Fe: 1×2 = 2
O: 1.5×2 = 3
- Empirical formula: Fe₂O₃
Problem 3:
A compound contains 2.7 g of aluminum (Al) and 2.4 g of oxygen (O). Find its empirical formula.
(Atomic masses: Al = 27, O = 16)
Solution:
- Convert mass to moles:
Moles of Al = 2.7/27 = 0.1
Moles of O = 2.4/16 = 0.15
- Divide by the smallest value (0.1):
Al: 0.1/0.1 = 1
O: 0.15/0.1 = 1.5
- Multiply to get whole numbers:
Al: 1×2 = 2
O: 1.5×2 = 3
- Empirical formula: Al₂O₃
Problem 4:
A compound is 87.5% nitrogen (N) and 12.5% hydrogen (H) by mass. Find its empirical formula.
(Atomic masses: N = 14, H = 1)
Solution:
- Convert percentages to moles:
Moles of N = 87.5/14 = 6.25
Moles of H = 12.5/1 = 12.5
- Divide by the smallest value (6.25):
N: 6.25/6.25 = 1
H: 12.5/6.25 = 2
- Empirical formula: NH₂
What is AMU?
AMU stands for Atomic Mass Unit. It is a small unit of mass used to measure the mass of atoms and molecules.
- Definition:
1 AMU is defined as one-twelfth (1/12) the mass of a carbon-12 atom.
In simple terms, it’s a way to express how heavy an atom is compared to a carbon atom.
- Why is AMU Used?
Atoms are extremely small, and their masses are too tiny to measure in grams. So, scientists use AMU to make it easier to compare atomic masses.
Key Points:
- Mass of Proton: ~1 AMU
- Mass of Neutron: ~1 AMU
- Mass of Electron: ~0.00055 AMU (negligible compared to protons and neutrons).
Examples of Atomic Mass in AMU:
Hydrogen (H): 1 AMU
Carbon (C): 12 AMU
Oxygen (O): 16 AMU
When we say “Oxygen has an atomic mass of 16 AMU,” it means it is 16 times heavier than 1/12 of a carbon-12 atom.
Fun Fact:
AMU is now officially called the Dalton (Da) in modern science, but the term AMU is still widely used in classrooms.
Example Problem:
How many molecules are present in 18 g of water?
Solution:
1 mole of water = molecules
Mass of 1 mole of water = 18 g
So, 18 g of water contains molecules.
Dalton’s Atomic Theory
Finally, let’s revisit Dalton’s Atomic Theory, which laid the foundation for our understanding of atoms:
1. Matter is made up of tiny indivisible particles called atoms.
2. Atoms of a given element are identical in mass and properties.
3. Atoms combine in fixed, whole-number ratios to form compounds.
4. Atoms are neither created nor destroyed in chemical reactions.
While Dalton’s theory had limitations (e.g., atoms are divisible into subatomic particles), it remains a cornerstone of chemistry.
Short Notes
Atoms are the smallest units of matter, and molecules are groups of atoms bonded together.
Chemical reactions follow the Laws of Conservation of Mass and Definite Proportions.
The mole concept simplifies the counting of atoms and molecules in bulk quantities.
Understanding chemical formulae and atomic mass is essential for representing and calculating chemical substances.
I hope you now have a clear understanding of how atoms and molecules form the basis of everything in the universe. If you have any questions, feel free to ask!







Enjoyed reading through this, very good stuff, regards.
I have recently started a site, the information you offer on this site has helped me greatly. Thank you for all of your time & work.
Wonderful beat ! I would like to apprentice while you amend your web site, how can i subscribe for a blog web site? The account helped me a applicable deal. I had been a little bit acquainted of this your broadcast offered bright clear idea
As I web site possessor I believe the content material here is rattling wonderful , appreciate it for your hard work. You should keep it up forever! Best of luck.
Fantastic beat ! I would like to apprentice even as you amend your site, how can i subscribe for a blog web site? The account helped me a appropriate deal. I had been tiny bit familiar of this your broadcast provided bright transparent idea
It’s actually a nice and useful piece of information. I am glad that you shared this useful information with us. Please keep us up to date like this. Thanks for sharing.
Merely a smiling visitant here to share the love (:, btw great style. “Reading well is one of the great pleasures that solitude can afford you.” by Harold Bloom.
I’m no longer certain where you are getting your information, however good topic. I must spend a while finding out more or figuring out more. Thanks for magnificent information I was searching for this info for my mission.
As a Newbie, I am always exploring online for articles that can be of assistance to me. Thank you
I found your weblog site on google and verify a couple of of your early posts. Continue to keep up the excellent operate. I just further up your RSS feed to my MSN Information Reader. Seeking forward to studying more from you afterward!…
of course like your website however you need to take a look at the spelling on several of your posts. Many of them are rife with spelling issues and I in finding it very bothersome to inform the reality then again I’ll surely come again again.
I got what you intend, thankyou for putting up.Woh I am thankful to find this website through google. “Do not be too timid and squeamish about your actions. All life is an experiment.” by Ralph Waldo Emerson.
I discovered your blog website on google and examine a few of your early posts. Continue to keep up the superb operate. I simply extra up your RSS feed to my MSN Information Reader. In search of ahead to studying extra from you later on!…
Good website! I truly love how it is easy on my eyes and the data are well written. I’m wondering how I could be notified whenever a new post has been made. I’ve subscribed to your RSS feed which must do the trick! Have a nice day!
I’m not that much of a internet reader to be honest but your sites really nice, keep it up! I’ll go ahead and bookmark your site to come back later on. Cheers
I believe this internet site holds some really superb info for everyone :D. “Nothing surely is so disgraceful to society and to individuals as unmeaning wastefulness.” by Count Benjamin Thompson Rumford.
It¦s really a great and helpful piece of info. I am happy that you shared this useful info with us. Please keep us informed like this. Thanks for sharing.
Some truly nice stuff on this internet site, I enjoy it.
You need to take part in a contest for the most effective blogs on the web. I’ll recommend this site!
Hi there! This post couldn’t be written any better! Reading through this post reminds me of my previous room mate! He always kept talking about this. I will forward this article to him. Pretty sure he will have a good read. Thank you for sharing!
Hello! I could have sworn I’ve been to this blog before but after browsing through some of the post I realized it’s new to me. Anyways, I’m definitely happy I found it and I’ll be book-marking and checking back frequently!
For dependable and expert roof installation services in Lancaster, Roof Installation Pros is your go-to choice. They specialize in all roofing types, delivering lasting solutions that safeguard your property and boost its value. Count on them for affordable, high-quality, and on-time service.
For dependable and expert roof installation services in Lancaster, Roof Installation Pros is your go-to choice. They specialize in all roofing types, delivering lasting solutions that safeguard your property and boost its value. Count on them for affordable, high-quality, and on-time service.
When it comes to roof installation in Lancaster, Roof Installation Pros stands out for quality and reliability. Their expert team works with all roof types, delivering strong, protective installations that add value. Highly rated for affordable, on-time, and professional roofing work.
Need top-notch roof installation in Lancaster? Roof Installation Pros delivers professional services with a skilled team ready to handle all roof types. Their work is reliable, durable, and adds value to your home—trusted by many for timely and budget-friendly roofing solutions.
Need top-notch roof installation in Lancaster? Roof Installation Pros delivers professional services with a skilled team ready to handle all roof types. Their work is reliable, durable, and adds value to your home—trusted by many for timely and budget-friendly roofing solutions.
Hi there! This post couldn’t be written any better! Reading through this post reminds me of my previous room mate! He always kept talking about this. I will forward this article to him. Pretty sure he will have a good read. Thank you for sharing!
I have been reading out many of your articles and it’s clever stuff. I will surely bookmark your blog.
Those are yours alright! . We at least need to get these people stealing images to start blogging! They probably just did a image search and grabbed them. They look good though!
Thank you, I’ve just been searching for info about this topic for ages and yours is the greatest I have came upon till now. However, what in regards to the conclusion? Are you sure in regards to the supply?
Good post. I learn something tougher on totally different blogs everyday. It would at all times be stimulating to read content material from other writers and observe slightly something from their store. I’d choose to use some with the content material on my weblog whether or not you don’t mind. Natually I’ll offer you a link in your web blog. Thanks for sharing.
Hey there! This post couldn’t be written any better! Reading through this post reminds me of my old room mate! He always kept chatting about this. I will forward this page to him. Fairly certain he will have a good read. Many thanks for sharing!
I will immediately snatch your rss feed as I can not to find your email subscription link or e-newsletter service. Do you’ve any? Please allow me recognise in order that I could subscribe. Thanks.
I dugg some of you post as I thought they were very useful handy
certainly like your website however you need to check the spelling on several of your posts. Several of them are rife with spelling issues and I in finding it very bothersome to tell the truth then again I?¦ll definitely come back again.
Would you be all for exchanging hyperlinks?
I really enjoy looking through on this web site, it holds excellent articles. “Never fight an inanimate object.” by P. J. O’Rourke.
Adorei este site. Para saber mais detalhes acesse o site e descubra mais. Todas as informações contidas são conteúdos relevantes e únicos. Tudo que você precisa saber está está lá.
Adorei este site. Pra saber mais detalhes acesse nosso site e descubra mais. Todas as informações contidas são informações relevantes e exclusivas. Tudo que você precisa saber está está lá.
Some truly prime articles on this website , bookmarked.
You have observed very interesting points! ps nice website.
Great blog! Is your theme custom made or did you download it from somewhere? A design like yours with a few simple tweeks would really make my blog stand out. Please let me know where you got your theme. Appreciate it
Very interesting topic, regards for posting. “Wrinkles should merely indicate where smiles have been.” by Mark Twain.
Excellent read, I just passed this onto a colleague who was doing a little research on that. And he actually bought me lunch because I found it for him smile Therefore let me rephrase that: Thanks for lunch! “There are places and moments in which one is so completely alone that one sees the world entire.” by Jules Renard.
I am always browsing online for ideas that can help me. Thank you!
I would like to thank you for the efforts you’ve put in writing this site. I’m hoping the same high-grade web site post from you in the upcoming also. Actually your creative writing skills has encouraged me to get my own website now. Really the blogging is spreading its wings quickly. Your write up is a good example of it.
Very interesting info!Perfect just what I was looking for!
I have been absent for a while, but now I remember why I used to love this web site. Thank you, I?¦ll try and check back more frequently. How frequently you update your site?
An interesting discussion is worth comment. I think that you should write more on this topic, it might not be a taboo subject but generally people are not enough to speak on such topics. To the next. Cheers
This really answered my problem, thank you!
A person essentially assist to make severely articles I might state. That is the very first time I frequented your web page and so far? I surprised with the analysis you made to make this actual publish incredible. Excellent job!
Discover the transformative power of businessiraq.com, Iraq’s premier B2B platform revolutionizing how businesses connect and thrive in the Middle East’s most dynamic market. Our comprehensive bilingual directory serves as the definitive bridge between Iraqi enterprises and global opportunities, offering real-time access to verified company profiles, financial metrics, and executive contacts across all major industries. Through our sophisticated platform, powered by partnerships with leading data providers including BoldData, we deliver unparalleled business intelligence and market insights that drive informed decision-making. Business owners can elevate their market presence through SEO-optimized listings while gaining access to our extensive network of over 50,000 registered companies, spanning from established corporations to emerging startups across all Iraqi governorates, including Kurdistan. At the heart of our platform lies a powerful suite of integrated tools designed to facilitate seamless business connections and market entry. From our live tender tracking system and interactive B2B matchmaking services to specialized market analysis reports and investment guides, businessiraq.com provides everything needed to navigate Iraq’s $200B+ economy successfully. Our commitment to excellence extends to innovative features including virtual business matchmaking events, industry-specific webinars, integrated translation services, and custom market research capabilities, all accessible through our mobile-responsive interface supporting both Arabic and English users. International investors and local businesses alike benefit from our comprehensive ecosystem of services, including company verification badges, expert consultation for market entry strategies, and real-time notifications of relevant business opportunities. By partnering with prestigious organizations like the Iraq Britain Business Council, we ensure our users have access to the latest trade opportunities and market developments. Whether you’re seeking reliable Iraqi suppliers, exploring investment possibilities, or looking to expand your business presence in Iraq, businessiraq.com stands as your trusted partner in achieving sustainable growth and success in one of the region’s most promising markets. Join today to unlock premium features and position your enterprise at the forefront of Iraq’s expanding business landscape, where opportunities for growth and collaboration await.
Revolutionizing market intelligence in Iraq, Businessiraq.com emerges as a game-changing platform by integrating artificial intelligence and data analytics into its Iraq business directory. The platform’s innovative Smart Match feature automatically connects businesses with compatible partners based on their profiles, industry focus, and business objectives. Real-time business news in Iraq is enhanced with predictive market analysis, helping companies anticipate trends and opportunities. The platform’s Iraq jobs section now includes skill-matching algorithms, while the tender directory offers automated alerts for relevant opportunities. With enhanced online business listings featuring virtual showrooms and 360-degree company tours, Businessiraq.com is transforming how businesses connect and thrive in Iraq’s digital age.
I have been absent for some time, but now I remember why I used to love this blog. Thanks , I¦ll try and check back more frequently. How frequently you update your website?
With a focus on precision and reliability, BWER offers state-of-the-art weighbridge systems to Iraq’s industries, meeting international standards and supporting operational efficiency.
Thanx sir .I am ayush saxena from DPS school Lucknow.